Electron Charge
On the elementary electrical charge and the Avogadro constant (1913)
The electromagnetic force dominates our day to day existence: high voltage powers the incessant hum or our factories and work places, the world wide web gives us access to an endless stream of data, electric lights have even allowed us to turn night into day. All these wonders are founded on to the movement of a tiny and inconspicuous electron, producing alternating electric and magnetic fields.
It will therefore be a surprise to some that the value of the electron’s charge was not known with certainty until well after the start of the industrial revolution.
Robert A. Millikan in his paper, On the Elementary Electrical Charge and the Avogadro Constant, presents a modified version of the oil drop experiment designed to determine the value of the elementary electric charge (e) and by extension Avogadro’s constant which relates to the number of particles or molecules found in a volume of gas at standard temperature and pressure.
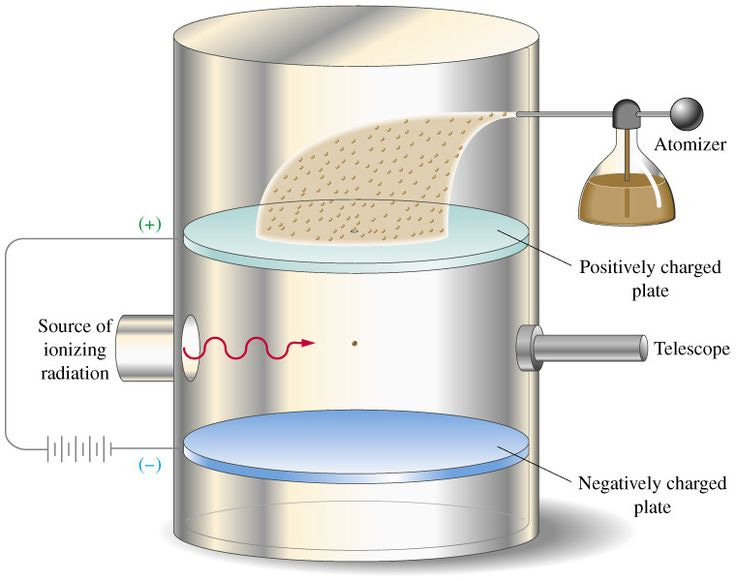
The Oil-Drop Method
The oil-drop experiment hinges on observing tiny oil droplets suspended between two electrically charged plates. By balancing the gravitational force pulling the droplet downward with an upward electric force one can measure the electric charge on individual droplets.
Taking into account additional factors such as viscosity and electric field strength, a formula for the elementary charge can be derived: