The Bohr Model
On the Constitution of Atoms and Molecules (1913)
In 1913, Danish physicist Niels Bohr conceived a seminal model of the atom, fundamentally altering our understanding of atomic structure and laying the foundation for quantum mechanics.
His paper, On the Constitution of Atoms and Molecules, takes us on a journey that merges classical mechanics with quantum principles, providing the first clear explanation of atomic behavior. Though a rather long paper by modern standards, its mathematics are quite simple as Bohr’s work predates some of the more mind-bending linear algebra typically associated with quantum mechanics.
Rutherford's Atom and Its Challenges
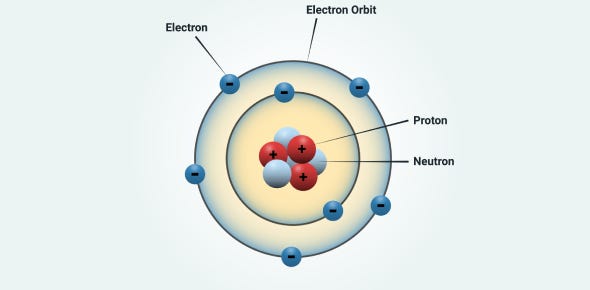
Bohr ultimately built upon Ernest Rutherford's atomic model, which described atoms as consisting of a dense, positively charged nucleus surrounded by electrons. While this model explained certain experimental findings, it left one glaring issue unresolved: according to classical electrodynamics, electrons in orbit around a nucleus would continuously emit radiation, spiraling inward until the atom collapsed. Clearly, this doesn’t happen, as atoms are remarkably stable.
Bohr addressed this discrepancy by introducing a novel idea. He proposed that electrons could occupy specific, stable orbits around the nucleus without radiating energy. These orbits, or "stationary states," were quantized, meaning only certain discrete energy levels were allowed.